 Understanding Clinical Trials
Understanding Clinical TrialsUnderstanding Clinical Trials
Interpreting clinical trial results
A clinical trial is also known as a study. You may have questions about how studies are organized and what the study results mean for a certain treatment.
One of the most reliable ways to show if a treatment works is to test it in a randomized controlled clinical trial. Here you’ll find information about why this study method is so reliable, as well as other helpful information about clinical studies.
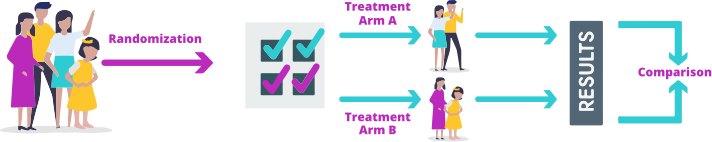
What is a randomized controlled clinical trial?
In a randomized controlled clinical trial, the participants are divided by chance (randomized) into different groups (treatment arms). Each group receives a different treatment. The results from each group are then compared. The trial may test 2 or more therapies at the same time, so it's possible to compare their effectiveness and safety. A nonrandomized controlled trial gives all patients the same therapy, so the results cannot be compared with another therapy.
Randomized controlled trial
Nonrandomized controlled trial

Why is it important to compare treatments?
A randomized controlled trial that compares treatment results is the preferred way to do a clinical trial because it aims to reduce the chance of bias for or against a treatment. This type of study means that every child has the same chance of being in a treatment arm and it helps the researchers compare the results fairly.
 Does it matter which patients are included in a study?
Does it matter which patients are included in a study?Does it matter which patients are included in a study?
Each study has guidelines about which patients can be in the study. Patients in a study can be different ages. They may have different weights and heights. They may be male or female or both. However, all the patients should have the same type or stage of disease and they should all have had the same or similar treatments before the study starts. This helps the researchers compare the results of the treatments in the study more effectively.
Does it matter how many patients are included in a study?
Generally, the more people enrolled in a study, the more reliable the results will be. The number of patients enrolled in a study is known as the sample size. A large sample size lets researchers more clearly see a difference between the treatment results.
Where do study patients come from?
Clinical studies can enroll patients from multiple institutions, such as hospitals, cancer care centers, or cancer clinics, or from a single institution. Multiple-institution studies are preferred because they help show if the results are the same across more patients in many different places in the United States and/or around the world.

How was the study treatment evaluated?
Randomized controlled trials may use different outcome measures to evaluate how well a new treatment works. Event-free survival (EFS), overall survival (OS), and objective response (OR) are all different ways that studies can measure how well a therapy is working. It is important to avoid comparing studies that use different outcome measures. You can read more about each below.
EFS
Event-free survival
What it measures
One of the most important measures of EFS is how long the patient stays in remission. For example, if a study reports a 3-year EFS of 50%, it means that 3 years after their study treatment began, 50% of the patients are alive and in remission.
Why it's important
- EFS shows how well the therapy can keep the patient in remission, meaning the cancer can no longer be detected
OS
Overall survival
What it measures
The length of time, after either the date of a cancer diagnosis or the start of treatment, that patients are still alive. For example, if a study reports a 5-year OS of 70%, it means that after 5 years, 70% of the patients are still alive.
Why it's important
- Unlike EFS, OS percentages show how many patients are alive after therapy; however, patients could have relapsed or received additional treatments during that time
OR
Objective response
What it measures
An objective response means a child had either a complete or partial response to the treatments they received.
Why it's important
- A complete response (CR) is the disappearance of all signs of cancer in response to treatment. However, this does not always mean the cancer has been cured
- A partial response (PR) is a decrease in the size of the tumor or the extent of cancer in the body
Understanding information about clinical trials may be difficult. You can find information from many sources, especially on the internet. However, some of the information may be misinformation. Ask your doctor to help you interpret the information so that you can make the right treatment decision for your child.
Understanding Clinical Trials
Hear from a pediatric oncologist discussing some of the confusing topics around understanding clinical trials and interpreting their results.